Michael Allan Physics and Chemistry with Free Electrons
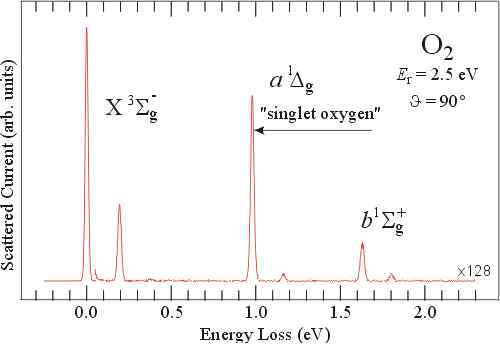
The Singlet Oxygen Spectrum
This Electron Energy Loss Spectrum of Molecular Oxygen shows the spin-forbidden transitions from the triplet ground state to the singlet excited states. The famous singlet oxygen is important in chemistry and biochemistry. It is extremely reactive and responsible, among others, for sunburn.
References:- M. Allan, Chimia 48 (1994) 372.
- M. Allan, J. Phys. B 28 (1995) 4329.
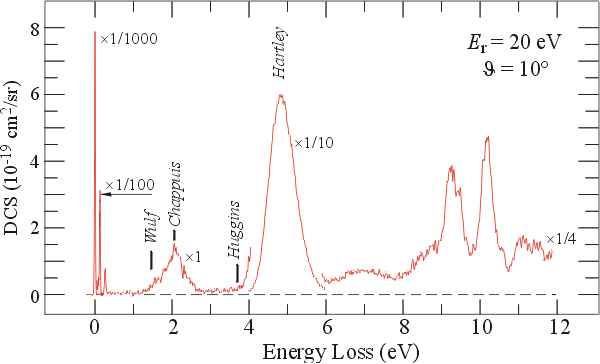
The "Ozone-hole" Spectrum
This Electron Energy Loss Spectrum of Ozone has been recorded at higher electron energies and a small scattering angle and therefore shows the spin and dipole allowed transitions. The Hartley band is the 'famous' absorption band which protects us from the harmful near UV radiation.
Reference:- Michael Allan, Nigel J. Mason and Julia A. Davies, J. Chem. Phys. 105 (1996) 5665
February 2020 (MA) (originally April 6, 2001)